MENU
FJ | FJD
FJ | FJD
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.
No results found
Search Suggestions
How do I Monitor the Growth Rate of My Microbial Culture?
Natascha Weiß Lab Academy
- Microbiology
- Lab Routine
- Culture of Microorganisms
- Photometry
- Essay
Can I proceed with my prokaryotic cells or do I still need to wait another hour? A sufficient growth rate is needed for microbial cells before you start to harvest or to start your main culture. A typical growth check can be done by OD600 with your photometer.
Microorganisms such as bacteria and yeast are employed in a multitude of experiments in microbiology and molecular biology laboratories. In order to propagate them, a liquid medium containing all necessary nutrients is typically inoculated with a colony or a starter culture and subsequently incubated for several hours. The incubation time depends on the organism, the culture conditions and the intended use of the culture.
Many applications require harvesting the cells during the exponential growth phase (log phase) in order to obtain the highest possible number of live cells. The right time for harvest may be based on experience; however, it is safer to monitor culture growth on a regular basis. One quick, simple and inexpensive method is to measure the optical density at 600 nm (OD600).
Molecules such as nucleic acids and proteins, which exist in solution, absorb light at defined wavelengths. A culture of microorganisms, on the other hand, constitutes a suspension, which absorbs very little light during photometric measurements. Instead, cells deflect the light, which is the reason that the OD600 method is also known as scattered light measurement or turbidimetry (figure 1). However, the measuring principle of both methods is the same in the photometer: Light is sent through a sample and the original light intensity is compared with the light intensity attenuated by the sample.
Many applications require harvesting the cells during the exponential growth phase (log phase) in order to obtain the highest possible number of live cells. The right time for harvest may be based on experience; however, it is safer to monitor culture growth on a regular basis. One quick, simple and inexpensive method is to measure the optical density at 600 nm (OD600).
Molecules such as nucleic acids and proteins, which exist in solution, absorb light at defined wavelengths. A culture of microorganisms, on the other hand, constitutes a suspension, which absorbs very little light during photometric measurements. Instead, cells deflect the light, which is the reason that the OD600 method is also known as scattered light measurement or turbidimetry (figure 1). However, the measuring principle of both methods is the same in the photometer: Light is sent through a sample and the original light intensity is compared with the light intensity attenuated by the sample.
Read more
Read less
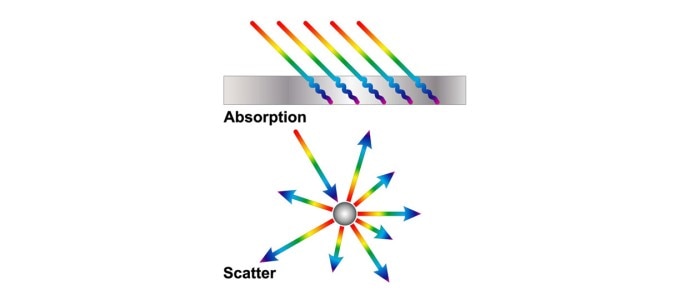
Figure 1: Two possible interactions of light with substances. In the case of absorption, the substance absorbs the light or part of it. Scatter results from the deflection of light in different directions.
Many instruction manuals for the culture of microorganisms in liquid media state the culture is to be harvested at an OD600 value. Such a value, however, is only meaningful in the context of the specific bacterial strain that is cultured under defined growth conditions and measured using a specific instrument.
The reason for such requirements is that, in contrast with absorbance measurements, the light scatter that occurs during turbidity measurements is strongly dependent on the size and shape of the cells as well as the optical design of the photometer [1, 2]. Different strains thus scatter light in different ways, and different growth conditions may also influence the size and the shape of a cell. The influence of the photometer becomes evident once the sample is measured using different instrument types; discrepancies as high as 100% of the measured OD600 values are not unheard of [2]. With respect to the intensity and the angle of incidence of the stray light that reaches the detector of the photometer, the distance between the aperture and the detector, as well as the size of the aperture behind the cuvette shaft, are of critical importance (figure 2) [2].
The reason for such requirements is that, in contrast with absorbance measurements, the light scatter that occurs during turbidity measurements is strongly dependent on the size and shape of the cells as well as the optical design of the photometer [1, 2]. Different strains thus scatter light in different ways, and different growth conditions may also influence the size and the shape of a cell. The influence of the photometer becomes evident once the sample is measured using different instrument types; discrepancies as high as 100% of the measured OD600 values are not unheard of [2]. With respect to the intensity and the angle of incidence of the stray light that reaches the detector of the photometer, the distance between the aperture and the detector, as well as the size of the aperture behind the cuvette shaft, are of critical importance (figure 2) [2].
Read more
Read less
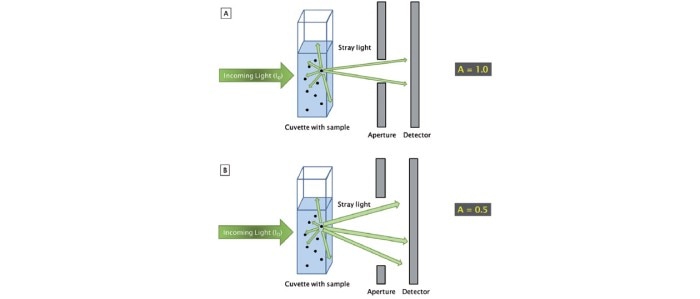
Figure 2: The different optical designs of photometers A and B lead to a change in the amount of light that reaches the detector and, as a consequence, to different results when measuring stray light from identical sample concentrations.
If the measured optical density is to be expressed in relation to a culture parameter such as, for example, the cell number, it is necessary to calibrate the photometer. In order to determine the concentration of the cells, OD600 values are aligned with the number of cells of the cultured strain. Otherwise, there is a possibility that aberrant measurement values may lead to a false interpretation of the growth phase of the culture, and in the worst case, the subsequent experiment may not be successfully carried out.
[1] Janke SA, Fortnagel P, Bergmann R. Microbiological turbidimetry using standard photometers. BIOspektrum, 1999; Vol. 6: 501-502.
[2] Harnack K, Spolaczyk R, Janke SA. Turbidity measurements (OD600) with absorption spectrometers. BIOspektrum, 1999; Vol. 6: 503-504.
References:
[1] Janke SA, Fortnagel P, Bergmann R. Microbiological turbidimetry using standard photometers. BIOspektrum, 1999; Vol. 6: 501-502.[2] Harnack K, Spolaczyk R, Janke SA. Turbidity measurements (OD600) with absorption spectrometers. BIOspektrum, 1999; Vol. 6: 503-504.
Read more
Read less

