MENÜ
CH | CHF
CH | CHF
-
- Tischzentrifugen
- Standzentrifugen
- Gekühlte Zentrifugen
- Mikrozentrifugen
- Mehrzweckzentrifugen
- Hochgeschwindigkeitszentrifugen
- Ultrazentrifugen
- Concentrator
- IVD Produkte
- High-Speed and Ultracentrifuge Consumables
- Zentrifugenröhrchen
- Zentrifugenplatten
- Gerätemanagement
- Proben- und Informationsmanagement
Es konnten keine Ergebnisse gefunden werden.
Such-Empfehlungen

Tired of Condensation in Your Tubes?
Jan Bebermeier Lab Academy
- Mixers & Shakers
- Essay
Heating up sample tubes in a thermoblock or dry heater may create condensation within the tube lids. As a result, your enzymatic reaction may be less efficient as expected. Counter-heating can avoid this.
Remember the last time when your tubes showed condensation droplets after temperature incubation in a thermoblock or thermomixer? Eventually, you even had a performance loss in your enzymatic reaction.
Condensation is defined as the change from the gas phase into the liquid phase. Condensation is the reverse process of vaporization. The condensation process occurs when the vapor is saturated and gets in contact with a cold (or colder) surface compared to its origin.
The higher the temperature delta between the warmest and the coldest spot within a closed system, the faster you will get condensation. The microtube is a perfectly closed system.
Many reactions need specific temperatures above room temperatures like 37°C, 42°C, 95°C, etc. As a result, an incubation step of 30 min at e.g. 65°C will end up with a layer of condensate droplets within the lid of your tube. You may think, one spin in the centrifuge and 100% of the sample are back at the bottom of the microtube. This process indeed helps to bring back the condensation droplets to your sample.
But there might still be an issue with the performed reaction which is done when you start your centrifugation step. Reagents need constant conditions for molecular processes like ligation, amplification, translation, restriction or whatever enzymatic reaction needs to be performed.
The reactivity of an enzyme depends on temperature, the availability of reaction partners but also on the salt concentration within the sample. Assuming a 25 µL enzymatic assay which has a salt concentration of e.g. 5 %. As the condensation process is limited to liquids, the calculated 3 µL condensate within the lid increases the salt concentration within the remaining sample at the bottom of the tube during the enzymatic reaction step by nearly 14 %.
(Rule of thumb: 5 % of 25 µL reaction volume: 1.25 µg salt, 22 µL of liquid incl. the salt remains after condensation, this is now 5.682 % of the total volume; resulting in 13.6 % higher salt concentration)
This increased salt concentration will probably not stop your reaction but might influence the efficiency of the enzyme, resulting in a different yield than possible under optimized conditions. Bottom line is, you are missing constant/ reproducible and efficient conditions.
There is a simple and convenient way to avoid condensation: Keep your sample liquids being the “coldest spot” in the reaction tube. For sure, the reaction temperature is set to e.g. 70°C. How to proceed?
One solution can be a counter-heating from the top of the vessel by a heated lid.
What is condensation?
Condensation is defined as the change from the gas phase into the liquid phase. Condensation is the reverse process of vaporization. The condensation process occurs when the vapor is saturated and gets in contact with a cold (or colder) surface compared to its origin.The higher the temperature delta between the warmest and the coldest spot within a closed system, the faster you will get condensation. The microtube is a perfectly closed system.
Many reactions need specific temperatures above room temperatures like 37°C, 42°C, 95°C, etc. As a result, an incubation step of 30 min at e.g. 65°C will end up with a layer of condensate droplets within the lid of your tube. You may think, one spin in the centrifuge and 100% of the sample are back at the bottom of the microtube. This process indeed helps to bring back the condensation droplets to your sample.
But there might still be an issue with the performed reaction which is done when you start your centrifugation step. Reagents need constant conditions for molecular processes like ligation, amplification, translation, restriction or whatever enzymatic reaction needs to be performed.
The reactivity of an enzyme depends on temperature, the availability of reaction partners but also on the salt concentration within the sample. Assuming a 25 µL enzymatic assay which has a salt concentration of e.g. 5 %. As the condensation process is limited to liquids, the calculated 3 µL condensate within the lid increases the salt concentration within the remaining sample at the bottom of the tube during the enzymatic reaction step by nearly 14 %.
(Rule of thumb: 5 % of 25 µL reaction volume: 1.25 µg salt, 22 µL of liquid incl. the salt remains after condensation, this is now 5.682 % of the total volume; resulting in 13.6 % higher salt concentration)
This increased salt concentration will probably not stop your reaction but might influence the efficiency of the enzyme, resulting in a different yield than possible under optimized conditions. Bottom line is, you are missing constant/ reproducible and efficient conditions.
There is a simple and convenient way to avoid condensation: Keep your sample liquids being the “coldest spot” in the reaction tube. For sure, the reaction temperature is set to e.g. 70°C. How to proceed?
One solution can be a counter-heating from the top of the vessel by a heated lid.
Mehr erfahren
Weniger lesen

Probably you know and use this concept in your daily work when using PCR machines. The lid of a cycler has a slightly higher temperature to avoid condensation by using an integrated heating system. In the first years of PCR, this prevention of condensation was done by using a layer of mineral oil on top of every sample.
As a completely integrated lid heating is far too complex for a thermoblock, a flexible heating cowling fulfills the same function.
As a completely integrated lid heating is far too complex for a thermoblock, a flexible heating cowling fulfills the same function.
Mehr erfahren
Weniger lesen
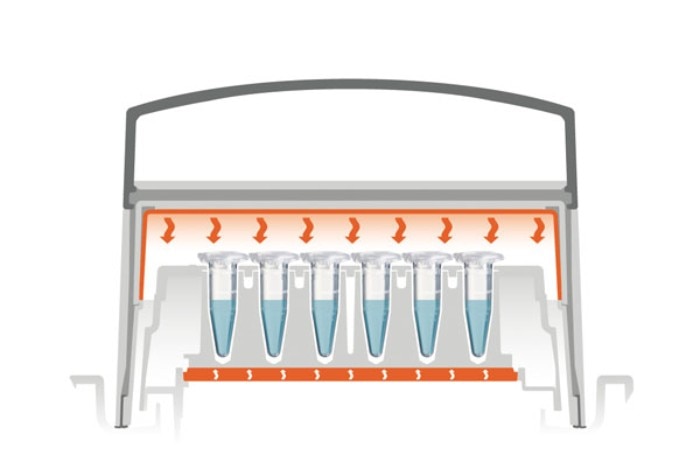
The heat cowling counter-heats from the top. A simple solution is an on/off-heating system which always provides a full power temperature. This solution may damage your samples as low temperature reactions at 30°C will be negatively affected by a 100 °C heat cowling.
The smart solution is a heat cowling which communicates with the thermoblock/ dry heater and adjusts the temperature for counter-heating to a few degrees above the block temperature.
The smart solution is a heat cowling which communicates with the thermoblock/ dry heater and adjusts the temperature for counter-heating to a few degrees above the block temperature.
Mehr erfahren
Weniger lesen

